What is the regulatory approach and what are its fundamental assumptions ?
Canada's regulatory approach is somewhat modeled on other OECD nations, although it has some relatively unique features that make our system less restrictive compared to the EU. The system was largely adapted from that used in chemical regulation, despite GE being a process with living organisms.
As identified above, not all aspects of this complex regulatory system are in place (despite approvals since the 1990s) and CEPA and its regulations act as the fall back authority. In general, the review of GE applications is done on a product by product basis, with several stages and data required by the applicant. Regulators do not typically generate their own data or require independent data generation or assessment. Data requirements are subject to significant regulatory interpretation since legislation and regulations are not very specific, without presription. Regulatory protocols and directives are somewhat more specific but are not necessarily publicly available for scrutiny. Depending on the nature of the application, reviews may be conducted by multiple government units. The different regulatory units attempt to co-ordinate their work since, for example, applications from a manufacturer for food, feed and plant approvals may all come forward at the same time, but be directed to different government units. There exists a "no-split approvals" policy to avoid circumstances where some uses are approved, others rejected. This is a result of the product by product assessment process and not having a harmonized GE legislative framework.
A preliminary phase in all cases is R&D in a laboratory setting conducted by the applicant. In some cases, approval for experimental work must be obtained. This phase can take many years and cost hundreds of millions of dollars. Once a developer (or an importer) wants to move the organism / product out of the laboratory and into trials, then different pathways are possible depending on the organism / product and what regulations are pertinent. Typically, if a GE organism requires field trials, an application must be made and data provided to the appropriate regulator for "confined" release. This data is then used to meet the data requirements for a later application for "unconfined" release. For GE foods, manufacturers or importers must file a notification of plans to sell or advertise a novel food. If the data provided with the notification is insufficient to determine safety, then additional data (determined on a product by product basis) may be required. Health Canada conducts the safety assessment if the original notice of intent to market requires it. However, the environmental assessment is conducted under the New Substances Notification Regulations of CEPA, unless the food is already regulated under the Seeds Act or Feeds Act as these are deemed to have CEPA equivalent environmental assessments. The New Substances Notification Regulations of CEPA are of two parts, one for chemicals, the other for organisms. Most foods / organisms are thus regulated under the Seeds or Feeds Acts, except those related specifically to micro-organisms. The regulator can reject, approve (through a letter of no objection), or approve with imposed conditions. In some cases, a product can also be deemed exempt from regulatory review. A lifecycle diagram for plants, feeds derived from plants, and food can be found here.
Key regulatory assumptions
Familiarity and substantial equivalence (adapted from CIELAP, 2002)
Familiarity is the main regulatory concept used to justify regulation of GE under existing legislation and regulation. It is founded on the vague idea that If the characteristics of an organism are known, the GE version does not differ except for its construct and we have experience of its use, then it can be deemed familiar, with confidence that there will be no adverse effects. Familiarity with the introduced trait, the environment, the organism and the interactions between them can all justify widespread release (Barrett and Abergel, 2000). Thus, justifying that a GE organism is familiar is fundamental to the entire review process. The contradiction is that because the entire regulatory system is based on the idea that they are familiar, it becomes much more challenging to assess their potential "unfamiliarity", to identify organism behaviour that is not consistent with the conventional analog (Clark, 1999). Most applications come forward on the presumption that they are familiar (with overtly "unfamiliar" applications not reaching the regulatory stage because the applicant knows it will trigger a full safety assessment), with minimal evidence to support the contention. This smacks heavily of circular reasoning and logical fallacy because the premises are often as unsupported as the conclusion. A consequence is that it is only post - release that problems appear because they have not necessarily been identified in the development and regulatory phase.
In concert with familiarity is substantial equivalence. Using information on conventional organisms or foods to establish the baseline for comparison, if the molecular, compositional and nutritional characteristics of both the GE organism or product and its conventional counterpart are comparable, then the GE organism or product is considered “substantially equivalent”. The term substantial is important because in comparisons of the conventional comparator with the GE organism or product, it is often the case that not all examined constituents fall into the same range (see for example the Decision Document on using GE salmon as animal feed). These divergences are usually excused as unsubstantial, in other words regulators don't deem them significant, which reveals the extent to which the system relies on regulator judgements about what is meaningful. The ecological performance of the comparator organism is often already problematic within conventional breeding and production regimes, but such problems have already been normalized and are essentially dismissed in the GE organism. This reality can contradict the expressed regulatory commitment to evidence-based assessment, in other words, human perceptions of what evidence is meaningful is in play here. When deemed substantially equivalent by regulators, the GE product / organism does not have to undergo safety and environmental testing beyond that used to determine whether substantial equivalence exists. This is the objective of the applicant, to demonstrate that the product is substantially equivalent to avoid additional safety testing.
However, the relationship between genetics, chemical composition, and toxicological and ecological risks is often not well known. The biochemical or toxicological effects of a GE product cannot be predicted from its chemical composition. Seemingly minor changes in foods (which typically exist) can have significant nutritional implications. If relationships are largely unknown, critics argue, how can similarity in composition be a predictor of equivalent ecological or toxicological behaviour (Millstone et al. 1999)?
Working together, these regulatory concepts assume that limited gene changes resulting from genetic engineering result in well-characterized responses, that knowledge of the conventional organism or food plus the GE trait is sufficient to predict future behavour. But it has been known for some time that single genes can affect many traits and produced unexpected expressions (Clark and Lehman, 2001; Heinemann et al, 2013). If the responses are often unpredictable, then substantial equivalence has no merit as a trigger for environmental and human health assessments. The Expert Panel of the Royal Society of Canada was particularly critical of the use of substantial equivalence as a decision threshold – the determination of whether a full risk assessment is required – and proposed that it be abandoned as a determination approach (An Expert Panel Report on the Future of Food Biotechnology 2001). Although regulatory theory suggests it should be otherwise, these concepts of familiarity and substantial equivalence are used in Canada as substitutes for environmental risk assessment (Barrett and Abergel, 2000).
Novel foods and feeds, and Plants with Novel Traits
An additional and unique part of the Canadian system is not to regulate GE per se, but rather plants, feeds and foods with novel traits, a much broader conception. This fits with the industry and decision-maker false equivalency narrative that GE is just a continuation of a millenia-old human project to biologically manipulate organisms for human benefit, that GE foods and organisms aren't really that different from their conventional analogs (cf. Andrée, 2002; Mueller and Flachs, 2022). While it is true humans have selected organisms for particular traits for thousands of years, and we have derived great benefits from doing so, this selection process was always undertaken within an ecological and socio-cultural context and the tools available for this manipulation were limited in scope, based primarily on observation of plants and animals in their environment. This meant that changes were slow, and fit broadly within the evolutionary patterns of organisms. This traditional manipulation of organisms could also be problematic, but its negative effects were limited by the specific environments in which it operated and the limited tools of manipulation and distribution. Genetic engineering, however, represents a dramatic shift in power, speed and ability to make fundamental changes to organisms outside of their ecological context, and globalized markets mean global reach. Particularly significant is the ability to cross species boundaries, by inserting sequences from one organism into another very rapidly. Until very recently, this was not done by genetic engineers with any real precision (despite their arguments to the contrary), and with limited appreciation of the implications for gene interactions.
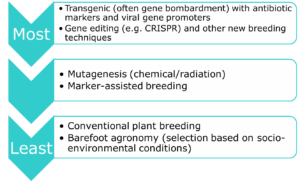
In the CFIA view, "A plant with a novel trait is a plant that contains a trait which is both new to the Canadian environment and has the potential to affect the specific use and safety of the plant with respect to the environment and human health. These traits can be introduced using biotechnology, mutagenesis, or conventional breeding techniques." In reality, certain manipulative techniques are more environmentally problematic than others, and therefore deserve more thorough consideration:
Novel foods according to Division 28 of Part B of the Food and Drug Regulations are ....
- "a substance, including a microorganism, that does not have a history of safe use as a food;
- a food that has been manufactured, prepared, preserved or packaged by a process that
- has not been previously applied to that food, and
- causes the food to undergo a major change; and
- a food that is derived from a plant, animal or microorganism that has been genetically modified such that
- the plant, animal or microorganism exhibits characteristics that were not previously observed in that plant, animal or microorganism,
- the plant, animal or microorganism no longer exhibits characteristics that were previously observed in that plant, animal or microorganism, or
- one or more characteristics of the plant, animal or microorganism no longer fall within the anticipated range for that plant, animal or microorganism. ( aliment nouveau)"
Novelty in a trait must be established for the regulatory review to be triggered, but the organism or the food or feed must also be substantially equivalent and familiar to avoid a full safety assessment. In other words, the novelty must be confined to the introduced trait itself.
The novel foods / novel plants designation obfuscates important distinctions in conventional vs. novel organism behaviour and makes scrutiny of regulatory decisions more challenging. The decision summaries released by regulators do not provide sufficient information for the public scrutiny of the decision making itself, particularly the regulator decisions about what variability in the GE organism can be dismissed as insignificant.
Data is supplied by the applicant, following protocols set forth by regulators
Regulators do not generate independent assessments, and it is not actually clear how often they utilize independent external scientific assessments, since most of these can only occur post - release of the GE organism / product. In other words, if there are problems, they are usually identified by independent scientists once the GE constructs are out in the world. Since regulatory decisions are not subject to independent scientific review, there are on going questions about the data quality of the industry submissions and the reviews undertaken by regulators. For one of the few Canadian cases where the data packages were made public post - release, both reviewers concluded that the data was inadequate to make a sound regulatory decision and that the submitted assessment would not pass a peer review (Barrett, 1999; Abergel, 2000). Similar data and testing protocol weaknesses, particularly related to limited proxy measures that don't adequately address possible impacts and relationships, poor or non-existent feeding trials, limited time and area trials, negative effects on non-target organisms, and statistical treatment of anomalous results, have been found in other jurisdictions when data packages or fuller decision documents have been made available (see citations in CIELAP, 2002; also Waltz, 2009; Bawa and Anilakumar, 2013; Landrigan and Benbrook, 2015; Hilbeck et al., 2015). These independent interpretations cast doubt on the health and environmental safety of many GE applications. That regulators operate in an institutional environment that wants GE applications on the market to fulfill government economic objectives has at least a subtle, if not overt, impact on how applications are interpreted (cf. Moran et al., 2009).
Evaluating the product, not the process
A very common broad regulatory concept in Canada regularly stated by decision makers is that the process by which a food is produced is not relevant, only the safety of the final product. This is somewhat like saying that how you make your money doesn't matter, it's only how much you make that's relevant. Certainly the tax and criminal justice systems don't believe that's true. And it isn't even an accurate description of the system in Canada because the novel foods regulations are triggered when, "food that has been manufactured, prepared, preserved or packaged by a process that has not been previously applied to that food". So the inconsistency in the regulation is that a novel food manufacturing process must be assessed but not a manipulation at the molecular level, surely a far more potentially serious novelty.
In the case of GE, the manipulation itself is in question. This has always been germane but now more so because of the new tools of gene editing (several techniques, including Clustered regularly interspaced short palindromic repeat, or CRISPR). One GE product of gene editing is currently on the market, a herbicide-tolerant canola, with several others in the pipeline. But the industry sees gene editing as the future, with most applications by 2030 from gene editing, because they find it cheaper and hope governments will simplify the regulatory process in the name of facilitating "innovation" (Arnason, 2020). Proponents claim that CRISPR applications can suppress fungal infections, parasites, and pests, provide resistance to droughts, eradicate weeds, increased photosynthesis and improve shelf life - all without the use of synthetic chemicals that are currently used in the dominant systems to achieve such results (cf. Clément and Ajena, 2021).
Although gene editing is more precise at the molecular level than earlier techniques, it is not necessarily more precise at the organism level, which means that how the changes are expressed may not be readily apparent within the limited time frames of application testing protocols. It has happened in the past, that a significant risk has emerged 20 years after the approval of GE applications (cf. Lantham and Wilson, 2013). A significant body of literature shows that genome editing can cause off-target effects in the genome, complex DNA re-arrangements and interference with gene regulation (see citations in Cotter and Peris, 2020 and Wilson, 2021), all of which can change organism behaviour, especially when considering gene-environment interactions which receive minimal attention from developers and regulators, in part because the testing protocols don't necessarily allow for such interactions to be revealed. In other words, gene editing is not as precise as claimed, as stated in a recent Nature Biotechnology editorial (Anon. 2020) and in a review of human genome editing precision by Bekaert et al. (2022). Part of the reason is the sheer number of genes, making it very difficult to know what to target, a challenge acknowledged by the industry (Arnason, 2020). The industry claims that the off-target effects are less problematic than those experienced with other breeding approaches, but this is an inappropriate baseline comparison given the problems of many other approaches and the failure to conduct plant breeding in an agroecological context (cf. Clément and Ajena, 2021).
For many applications, gene editing also still relies on first generation GE techniques for the development process, so the limitations of first generation techniques are also often applicable. Despite this evidence, in 2022, Health Canada (HC) (and AAFC in 2023), under pressure from and with the active collaboration of the biotechnology industry behind the scenes (see Gerbet, 2023), adopted new regulations to effectively exclude gene editing technologies from the novel foods / novel traits framework (Novel Foods Regulations, 2006, amended 2022; and amended 2023 Directive 2009-09: Plants with novel traits). Gene editing, thus, is no longer subject to regulatory oversight unless it contravenes certain regulatory assumptions about the technology, unlikely to occur since the biotechnology industry knows that this is the bargain struck. In so doing, the federal government has decided that biotechnology firms are the experts, despite ample evidence that they have limited understanding of the organism, population and ecological implications of their applications. Proponents and regulators appear to believe that gene editing will often meet those exemption conditions. It appears that a CRISPR derived camelina plant that produces oils for biodiesel production will be the first deregulated product on the market.
Canada once again finds itself pursuing a less robust regulatory pathway compared to Europe, where, under a European Court of Justice ruling in 2018, products developed by gene editing and related technologies are still subject to GE regulations. EU proposals to member states to permit certain products of gene editing are under consideration as of early 2024, but many gene editing techniques will still be assessed under existing GE regulations.
No consideration of scale
States Benbrook (2016), "It is assumed that the risks arising from the planting of any particular field to a GE crop will be determined solely by what happens in that field. Current risk assessments do not take into account whether a given GE technology is likely to be adopted on 1%, or 10%, or nearly 100% of the cropland planted to a specific crop." This is embedded in the assessment process in that industry and regulators assume that what happens on a small plot basis during research trials is an accurate reflection of what happens on a larger scale post-release. Any issues around degree of adoption post approval are considered market, not regulatory, matters. Many of these potential problems with GE on a large scale are compounded by our general weakness in determining landscape level impacts of food systems, particularly landscape simplification and biodiversity loss (see Goal 2). Marvier and van Acker (2005) considered transgene movement a certain outcome of widespread adoption through such mechanisms as gene flow during cultivation, volunteer crop populations, seed transport and human errors in handling.
Roundup Ready (RR) canola provides an important lesson in that widespread adoption has resulted in volunteer RR canola now being a top 5 weed problem on the Prairies that has to be controlled with herbicides other than Roundup. A majority of farmers are not following recommendations to only grow canola on any piece of land 1 year in 4 to minimize pest pressures. The regulatory review would not have adequately examined such possibilities. Most scientists working on development and review did not have significant training in ecology and thus could not anticipate the impacts of widespread adoption. In contrast, in agroecological frameworks, scale is a vital consideration because of how it affects many ecological processes (Altieri, 1987; Gliessman, 1990; Wojkowski, 2004; Lovell et al., 2010).
There are also important lessons from the termination of efforts to commercialize GE flax and GE wheat. In both cases, the end of field trials was supposed to assure no presence of the transgenes in the crop, but the transgenes were later found in batches significant enough to case markets to stop importing. This speaks to the inability to control gene flow across landscapes once planted.
Gene drive technology, based on gene editing, is perhaps the ultimate expression of this inattention to landscape level impacts. Currently in R&D stages and mostly targeted to insects and weeds, organisms are modified so when released in the wild, they drive genetic changes (often affecting reproduction) through an entire wild population, essentially irreversible, and potentially resulting in the deliberate extinction of some organisms. Regulatory systems have no way currently to assess the landscape level impacts of such events (see Cotter and Peris, 2020).
No mandatory consumer labeling
Voluntary labeling is permitted as long as it is not misleading. At this point it appears that no firms have labelled a food as a product of GE, but many use non-GE claims, some certified by the Non-GMO Project. No mandatory labeling means no provider of ingredients is obliged to reveal production method. This means that market signals can only be used to determine preferences or not for GE products.The absence of labels also means no post-consumption health assessments of consuming GE foods are possible. As discussed in Introduction, there is no assessment of broader societal benefit or harm, that is deemed a responsibility of the market. Other jurisdictions are more demanding in this regard, for example "the European Union (EU) regulatory approach includes socio-economic and ethical issues in the evaluation process and relies on the precautionary principle as the decision threshold during the evaluative process." (Moran et al. 2009:8) But in the absence of labeling, there are really no effective market signals for consumers and no way to track societal impacts.
No statutory compensation regimes for harm
"Canadian jurisdictions have not enacted statutory compensation regimes for harms associated with GM crops, so “liability flowing from GM activities must be assessed through the common law of torts.” (Moran et al., 2009:4). Since those most likely to be claiming harm are farmers who cannot grow their crops without risk of contamination and associated loss of markets, this approach limits possible claims since few are able to afford legal action. Small to medium distributors may also be similarly affected by supply chain contamination events.
Summary
What is clear from all these regulatory concepts and decisions is that transparency is limited (see also CBAN, 2022), especially in light of the decision to exclude gene editing from oversight. There are no real market signals by which consumers can make informed decisions and there is limited parliamentary involvement meaning that citizens cannot effectively express concerns through traditional political processes. The system is designed to minimize the likelihood of GE applications being rejected at the regulatory stage. All these regulatory assumptions are only tenable in an environment in which broader societal and environmental value of a technology is not examined, which is of course why it is not, because if it were, it would call into question all these assumptions. From all this it can only be concluded that Canada's regulatory system is profoundly ecological inept.